This information is intended for U.S. healthcare professionals only.

If you have adult patients living with the rarest forms of generalized myasthenia gravis
They may qualify to participate in the ADAPT SERON clinical study

Who Can Join?
- MuSK-Ab
- LRP4-Ab
- gMG patients with no identifiable autoantibodies
Trial Size
Study Drug
Efgartigimod is being evaluated for severe autoimmune diseases mediated by pathogenic IgG autoantibodies, including gMG.
The investigational study drug, efgartigimod, is not approved by the FDA for the treatment of patients with AChR-Ab seronegative gMG as efficacy and safety have not been established.

KEY Eligibility Criteria
- Patient is an adult
- Patient is not pregnant or plans to become pregnant
- Patient has no known weakness in infancy and later develop fatigable weakness after aged 16 years and diagnosed with acquired gMG confirmed by documentation of both of the following:
- – HHistory of abnormal neuromuscular transmission demonstrated by single fiber electromyography or RNS or is MuSK-Ab seropositive
- – Either a history of positive edrophonium chloride test OR a demonstrated improvement in MG signs with treatments such as oral AChE inhibitors, PLEX, immunoabsorption, or IVIg/SCIg treatment
- Patient is AChR-Ab seronegative at screening
- Patient is MGFA Class II, III, or IV
- Patient has a screening and baseline MG-ADL total score ≥5 points with >50% of the score attributed to non-ocular symptoms
- Patient is receiving a stable dose of MG therapy before screening that includes AChE inhibitors, steroids, or NSISTs in combination or alone, with the following dose conditions:
- – Nonsteroidal immunosuppressive drugs (e.g., azathioprine, methotrexate, cyclosporine, tacrolimus, mycophenolate mofetil, and cyclophosphamide) initiated at least 6 months before screening with no change in dose during the 3 months before screening
- – Steroids initiated at least 3 months before screening, with no change in dose during the month before screening
- – AChE inhibitors with no change in dose during the 2 weeks before screening
See if your patients can be part of this important study
The ADAPT SERON study
will be conducted at sites across the United States
| Site | Location |
|---|---|
| Neurology Offices of South Florida |
9970 Central Park Blvd N., Ste. 207 Boca Raton, FL 33428 |
| University of South Florida | 13330 USF Laurel Drive Tampa, FL 33612 |
| National Neuromuscular Research Institute |
4705 Spicewood Springs Road, Ste. 200 Austin, TX 78759 |
| Medsol Clinical Research Center | 4161 Tamiami Trl Ste. 120 Port Charlotte, FL 33952 |
| SFM Clinical Research, LLC | 1601 Clint Moore Rd., Ste. 120 Boca Raton, FL 33487 |
| University of North Carolina at Chapel Hill | 101 Manning Dr Chapel Hill, NC 27514 |
| University of Kansas Medical Center Research Institute, Inc. | 4300 Shawnee Mission Pkwy., Ste. 3340 Fairway, KS 66205 |
| Baycare Medical Group | 1201 5th Ave N Saint Petersburg, FL 33705 |
| Neurology Associates PA | 331 N Maitland Ave Dept A-1 Maitland, FL 32751 |
| Duke Early Phase Clinical Research Unit | 40 Duke Medicine Cir Durham, NC 27710 |
| Augusta University Medical Center | 1120 15th St Augusta, GA 30912 |
| Northwestern Memorial Hospital | 259 E Erie St Fl 19 Chicago, IL 60611 |
| HonorHealth Neurology - Bob Bové Neuroscience Institute | 350 W Thomas Rd Phoenix, AZ 85013 |
| Henry Ford Health System | 2799 W Grand Blvd Detroit, MI 48202 |
| Erlanger Health System | 975 E 3rd St Chattanooga, TN 37403 |
| Healthcare Innovations Institute, LLC | 5441 N University Dr Coral Springs, FL 33067 |
Study Design
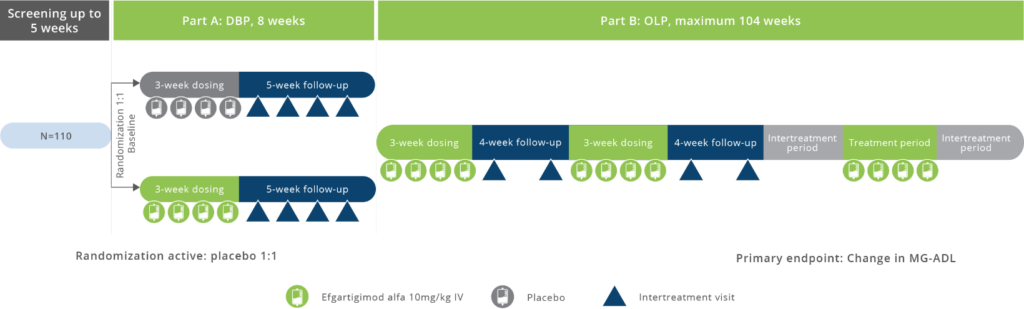
AcurianHealth helps connect people with research studies that offer treatment under development.
Since 1998, AcurianHealth has referred 1 million study candidates to 800 studies in 70 countries.
*In a research study, the participants may receive investigational study product or may receive an inactive substance, or placebo, depending on the study design. Participants receive study-related care from a doctor/research team for the duration of the study. For studies that offer compensation, reasonable payments will be made for participation. The length of the study may vary.
The investigational study drug, efgartigimod, is not approved by the FDA for the treatment of patients with AChR-Ab seronegative gMG as efficacy and safety have not been established.
©2024 Acurian, Inc. All rights reserved.
MED-US-EFG-2400100